
Big Sugar's Sweet Little Lies
Gary Taubes and Cristin Kearns Couzens
How the industry kept scientists from asking: Does sugar kill?
 Illustration: Chris Buzelli
Illustration: Chris Buzelli
On a brisk spring Tuesday in 1976, a pair of executives from the Sugar Association stepped up to the podium of a Chicago ballroom to accept the Oscar of the public relations world, the Silver Anvil award for excellence in "the forging of public opinion." The trade group had recently pulled off one of the greatest turnarounds in PR history. For nearly a decade, the sugar industry had been buffeted by crisis after crisis as the media and the public soured on sugar and scientists began to view it as a likely cause of obesity, diabetes, and heart disease. Industry ads claiming that eating sugar helped you lose weight had been called out by the Federal Trade Commission, and the Food and Drug Administration had launched a review of whether sugar was even safe to eat. Consumption had declined 12 percent in just two years, and producers could see where that trend might lead. As John "JW" Tatem Jr. and Jack O'Connell Jr., the Sugar Association's president and director of public relations, posed that day with their trophies, their smiles only hinted at the coup they'd just pulled off.
Their winning campaign, crafted with the help of the prestigious public relations firm Carl Byoir & Associates, had been prompted by a poll showing that consumers had come to see sugar as fattening, and that most doctors suspected it might exacerbate, if not cause, heart disease and diabetes. With an initial annual budget of nearly $800,000 ($3.4 million today) collected from the makers of Dixie Crystals, Domino, C&H, Great Western, and other sugar brands, the association recruited a stable of medical and nutritional professionals to allay the public's fears, brought snack and beverage companies into the fold, and bankrolled scientific papers that contributed to a "highly supportive" FDA ruling, which, the Silver Anvil application boasted, made it "unlikely that sugar will be subject to legislative restriction in coming years."
The story of sugar, as Tatem told it, was one of a harmless product under attack by "opportunists dedicated to exploiting the consuming public." Over the subsequent decades, it would be transformed from what the New York Times in 1977 had deemed "a villain in disguise" into a nutrient so seemingly innocuous that even the American Heart Association and the American Diabetes Association approved it as part of a healthy diet. Research on the suspected links between sugar and chronic disease largely ground to a halt by the late 1980s, and scientists came to view such pursuits as a career dead end. So effective were the Sugar Association's efforts that, to this day, no consensus exists about sugar's potential dangers. The industry's PR campaign corresponded roughly with a significant rise in Americans' consumption of "caloric sweeteners," including table sugar (sucrose) and high-fructose corn syrup (HFCS). This increase was accompanied, in turn, by a surge in the chronic diseases increasingly linked to sugar. Since 1970, obesity rates in the United States have more than doubled, while the incidence of diabetes has more than tripled. (The chart below uses sugar "availability" numbers rather than the USDA's speculative new consumption figures.)
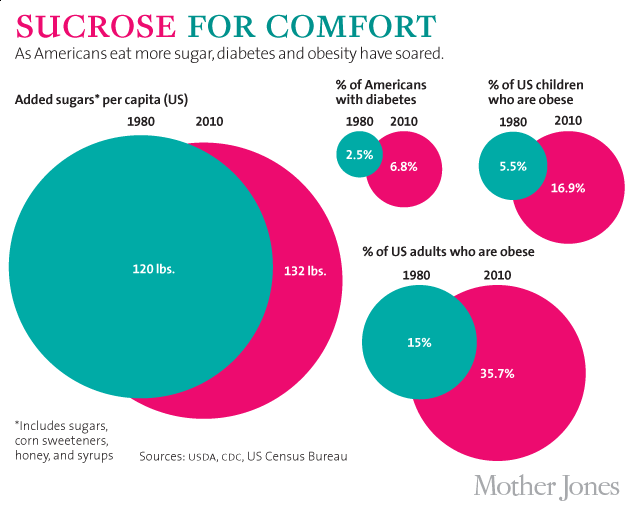
Precisely how did the sugar industry engineer its turnaround? The answer is found in more than 1,500 pages of internal memos, letters, and company board reports we discovered buried in the archives of now-defunct sugar companies as well as in the recently released papers of deceased researchers and consultants who played key roles in the industry's strategy. They show how Big Sugar used Big Tobacco-style tactics to ensure that government agencies would dismiss troubling health claims against their products. Compared to the tobacco companies, which knew for a fact that their wares were deadly and spent billions of dollars trying to cover up that reality, the sugar industry had a relatively easy task. With the jury still out on sugar's health effects, producers simply needed to make sure that the uncertainty lingered. But the goal was the same: to safeguard sales by creating a body of evidence companies could deploy to counter any unfavorable research.
This decades-long effort to stack the scientific deck is why, today, the USDA's dietary guidelines only speak of sugar in vague generalities. ("Reduce the intake of calories from solid fats and added sugars.") It's why the FDA insists that sugar is "generally recognized as safe" despite considerable evidence suggesting otherwise. It's why some scientists' urgent calls for regulation of sugary products have been dead on arrival, and it's why—absent any federal leadership—New York City Mayor Michael Bloomberg felt compelled to propose a ban on oversized sugary drinks that passed in September.
In fact, a growing body of research suggests that sugar and its nearly chemically identical cousin, HFCS, may very well cause diseases that kill hundreds of thousands of Americans every year, and that these chronic conditions would be far less prevalent if we significantly dialed back our consumption of added sugars. Robert Lustig, a leading authority on pediatric obesity at the University of California-San Francisco (whose arguments Gary explored in a 2011 New York Times Magazine cover story), made this case last February in the prestigious journal Nature. In an article titled "The Toxic Truth About Sugar," Lustig and two colleagues observed that sucrose and HFCS are addictive in much the same way as cigarettes and alcohol, and that overconsumption of them is driving worldwide epidemics of obesity and type 2 diabetes (the type associated with obesity). Sugar-related diseases are costing America around $150 billion a year, the authors estimated, so federal health officials need to step up and consider regulating the stuff.
The Sugar Association dusted off what has become its stock response: The Lustig paper, it said, "lacks the scientific evidence or consensus" to support its claims, and its authors were irresponsible not to point out that the full body of science "is inconclusive at best." This inconclusiveness, of course, is precisely what the Sugar Association has worked so assiduously to maintain. "In confronting our critics," Tatem explained to his board of directors back in 1976, "we try never to lose sight of the fact that no confirmed scientific evidence links sugar to the death-dealing diseases. This crucial point is the lifeblood of the association."
The Sugar Association's earliest incarnation dates back to 1943, when growers and refiners created the Sugar Research Foundation to counter World War II sugar-rationing propaganda—"How Much Sugar Do You Need? None!" declared one government pamphlet. In 1947, producers rechristened their group the Sugar Association and launched a new PR division, Sugar Information Inc., which before long was touting sugar as a "sensible new approach to weight control." In 1968, in the hope of enlisting foreign sugar companies to help defray costs, the Sugar Association spun off its research division as the International Sugar Research Foundation. "Misconceptions concerning the causes of tooth decay, diabetes, and heart problems exist on a worldwide basis," explained a 1969 ISRF recruiting brochure.
As early as 1962, internal Sugar Association memos had acknowledged the potential links between sugar and chronic diseases, but at the time sugar executives had a more pressing problem: Weight-conscious Americans were switching in droves to diet sodas—particularly Diet Rite and Tab—sweetened with cyclamate and saccharin. From 1963 through 1968, diet soda's share of the soft-drink market shot from 4 percent to 15 percent. "A dollar's worth of sugar," ISRF vice president and research director John Hickson warned in an internal review, "could be replaced with a dime's worth" of sugar alternatives. "If anyone can undersell you nine cents out of 10," Hickson told the New York Times in 1969, "you'd better find some brickbat you can throw at him."
By then, the sugar industry had doled out more than $600,000 (about $4 million today) to study every conceivable harmful effect of cyclamate sweeteners, which are still sold around the world under names like Sugar Twin and Sucaryl. In 1969, the FDA banned cyclamates in the United States based on a study suggesting they could cause bladder cancer in rats. Not long after, Hickson left the ISRF to work for the Cigar Research Council. He was described in a confidential tobacco industry memo as a "supreme scientific politician who had been successful in condemning cyclamates, on behalf of the [sugar industry], on somewhat shaky evidence." It later emerged that the evidence suggesting that cyclamates caused cancer in rodents was not relevant to humans, but by then the case was officially closed. In 1977, saccharin, too, was nearly banned on the basis of animal results that would turn out to be meaningless in people.
Meanwhile, researchers had been reporting that blood lipids—cholesterol and triglycerides in particular—were a risk factor in heart disease. Some people had high cholesterol but normal triglycerides, prompting health experts to recommend that they avoid animal fats. Other people were deemed "carbohydrate sensitive," with normal cholesterol but markedly increased triglyceride levels. In these individuals, even moderate sugar consumption could cause a spike in triglycerides. John Yudkin, the United Kingdom's leading nutritionist, was making headlines with claims that sugar, not fat, was the primary cause of heart disease.
In 1967, the Sugar Association's research division began considering "the rising tide of implications of sucrose in atherosclerosis." Before long, according to a confidential 1970 review of industry-funded studies, the newly formed ISRF was spending 10 percent of its research budget on the link between diet and heart disease. Hickson, the ISRF's vice president, urged his member corporations to keep the results of the review under wraps. Of particular concern was the work of a University of Pennsylvania researcher on "sucrose sensitivity," which sugar executives feared was "likely to reveal evidence of harmful effects." One ISRF consultant recommended that sugar companies get to the truth of the matter by sponsoring a full-on study. In what would become a pattern, the ISRF opted not to follow his advice. Another ISRF-sponsored study, by biochemist Walter Pover of the University of Birmingham, in England, had uncovered a possible mechanism to explain how sugar raises triglyceride levels. Pover believed he was on the verge of demonstrating this mechanism "conclusively" and that 18 more weeks of work would nail it down. But instead of providing the funds, the ISRF nixed the project, assessing its value as "nil."
The industry followed a similar strategy when it came to diabetes. By 1973, links between sugar, diabetes, and heart disease were sufficiently troubling that Sen. George McGovern of South Dakota convened a hearing of his Select Committee on Nutrition and Human Needs to address the issue. An international panel of experts—including Yudkin and Walter Mertz, head of the Human Nutrition Institute at the Department of Agriculture—testified that variations in sugar consumption were the best explanation for the differences in diabetes rates between populations, and that research by the USDA and others supported the notion that eating too much sugar promotes dramatic population-wide increases in the disease. One panelist, South African diabetes specialist George Campbell, suggested that anything more than 70 pounds per person per year—about half of what is sold in America today—would spark epidemics.
In the face of such hostile news from independent scientists, the ISRF hosted its own conference the following March, focusing exclusively on the work of researchers who were skeptical of a sugar/diabetes connection. "All those present agreed that a large amount of research is still necessary before a firm conclusion can be arrived at," according to a conference review published in a prominent diabetes journal. In 1975, the foundation reconvened in Montreal to discuss research priorities with its consulting scientists. Sales were sinking, Tatem reminded the gathered sugar execs, and a major factor was "the impact of consumer advocates who link sugar consumption with certain diseases."
Following the Montreal conference, the ISRF disseminated a memo quoting Errol Marliss, a University of Toronto diabetes specialist, recommending that the industry pursue "well-designed research programs" to establish sugar's role in the course of diabetes and other diseases. "Such research programs might produce an answer that sucrose is bad in certain individuals," he warned. But the studies "should be undertaken in a sufficiently comprehensive way as to produce results. A gesture rather than full support is unlikely to produce the sought-after answers."
A gesture, however, is what the industry would offer. Rather than approve a serious investigation of the purported links between sucrose and disease, American sugar companies quit supporting the ISRF's research projects. Instead, via the Sugar Association proper, they would spend roughly $655,000 between 1975 and 1980 on 17 studies designed, as internal documents put it, "to maintain research as a main prop of the industry's defense." Each proposal was vetted by a panel of industry-friendly scientists and a second committee staffed by representatives from sugar companies and "contributing research members" such as Coca-Cola, Hershey's, General Mills, and Nabisco. Most of the cash was awarded to researchers whose studies seemed explicitly designed to exonerate sugar. One even proposed to explore whether sugar could be shown to boost serotonin levels in rats' brains, and thus "prove of therapeutic value, as in the relief of depression," an internal document noted.
At best, the studies seemed a token effort. Harvard Medical School professor Ron Arky, for example, received money from the Sugar Association to determine whether sucrose has a different effect on blood sugar and other diabetes indicators if eaten alongside complex carbohydrates like pectin and psyllium. The project went nowhere, Arky told us recently. But the Sugar Association "didn't care."
In short, rather than do definitive research to learn the truth about its product, good or bad, the association stuck to a PR scheme designed to "establish with the broadest possible audience—virtually everyone is a consumer—the safety of sugar as a food." One of its first acts was to establish a Food & Nutrition Advisory Council consisting of a half-dozen physicians and two dentists willing to defend sugar's place in a healthy diet, and set aside roughly $60,000 per year (more than $220,000 today) to cover its cost.
Working to the industry's recruiting advantage was the rising notion that cholesterol and dietary fat—especially saturated fat—were the likely causes of heart disease. (Tatem even suggested, in a letter to the Times Magazine, that some "sugar critics" were motivated merely by wanting "to keep the heat off saturated fats.") This was the brainchild of nutritionist Ancel Keys, whose University of Minnesota laboratory had received financial support from the sugar industry as early as 1944. From the 1950s through the 1980s, Keys remained the most outspoken proponent of the fat hypothesis, often clashing publicly with Yudkin, the most vocal supporter of the sugar hypothesis—the two men "shared a good deal of loathing," recalled one of Yudkin's colleagues.
So when the Sugar Association needed a heart disease expert for its Food & Nutrition Advisory Council, it approached Francisco Grande, one of Keys' closest colleagues. Another panelist was University of Oregon nutritionist William Connor, the leading purveyor of the notion that it is dietary cholesterol that causes heart disease. As its top diabetes expert, the industry recruited Edwin Bierman of the University of Washington, who believed that diabetics need not pay strict attention to their sugar intake so long as they maintained a healthy weight by burning off the calories they consumed. Bierman also professed an apparently unconditional faith that it was dietary fat (and being fat) that caused heart disease, with sugar having no meaningful effect.
It is hard to overestimate Bierman's role in shifting the diabetes conversation away from sugar. It was primarily Bierman who convinced the American Diabetes Association to liberalize the amount of carbohydrates (including sugar) it recommended in the diets of diabetics, and focus more on urging diabetics to lower their fat intake, since diabetics are particularly likely to die from heart disease. Bierman also presented industry-funded studies when he coauthored a section on potential causes for a National Commission on Diabetes report in 1976; the document influences the federal diabetes research agenda to this day. Some researchers, he acknowledged, had "argued eloquently" that consumption of refined carbohydrates (such as sugar) is a precipitating factor in diabetes. But then Bierman cited five studies—two of them bankrolled by the ISRF—that were "inconsistent" with that hypothesis. "A review of all available laboratory and epidemiologic evidence," he concluded, "suggests that the most important dietary factor in increasing the risk of diabetes is total calorie intake, irrespective of source."
The point man on the industry's food and nutrition panel was Frederick Stare, founder and chairman of the department of nutrition at the Harvard School of Public Health. Stare and his department had a long history of ties to Big Sugar. An ISRF internal research review credited the sugar industry with funding some 30 papers in his department from 1952 through 1956 alone. In 1960, the department broke ground on a new $5 million building funded largely by private donations, including a $1 million gift from General Foods, the maker of Kool-Aid and Tang.
By the early 1970s, Stare ranked among the industry's most reliable advocates, testifying in Congress about the wholesomeness of sugar even as his department kept raking in funding from sugar producers and food and beverage giants such as Carnation, Coca-Cola, Gerber, Kellogg, and Oscar Mayer. His name also appears in tobacco documents, which show that he procured industry funding for a study aimed at exonerating cigarettes as a cause of heart disease.
The first act of the Food & Nutrition Advisory Council was to compile "Sugar in the Diet of Man," an 88-page white paper edited by Stare and published in 1975 to "organize existing scientific facts concerning sugar." It was a compilation of historical evidence and arguments that sugar companies could use to counter the claims of Yudkin, Stare's Harvard colleague Jean Mayer, and other researchers whom Tatem called "enemies of sugar." The document was sent to reporters—the Sugar Association circulated 25,000 copies—along with a press release headlined "Scientists dispel sugar fears." The report neglected to mention that it was funded by the sugar industry, but internal documents confirm that it was.
The Sugar Association also relied on Stare to take its message to the people: "Place Dr. Stare on the AM America Show" and "Do a 3 ½ minute interview with Dr. Stare for 200 radio stations," note the association's meeting minutes. Using Stare as a proxy, internal documents explained, would help the association "make friends with the networks" and "keep the sugar industry in the background." By the time Stare's copious conflicts of interest were finally revealed—in "Professors on the Take," a 1976 exposé by the Center for Science in the Public Interest—Big Sugar no longer needed his assistance. The industry could turn to an FDA document to continue where he'd left off.
While Stare and his colleagues had been drafting "Sugar in the Diet of Man," the FDA was launching its first review of whether sugar was, in the official jargon, generally recognized as safe (GRAS), part of a series of food-additive reviews the Nixon administration had requested of the agency. The FDA subcontracted the task to the Federation of American Societies of Experimental Biology, which created an 11-member committee to vet hundreds of food additives from acacia to zinc sulfate. While the mission of the GRAS committee was to conduct unbiased reviews of the existing science for each additive, it was led by biochemist George W. Irving Jr., who had previously served two years as chairman of the scientific advisory board of the International Sugar Research Foundation. Industry documents show that another committee member, Samuel Fomon, had received sugar-industry funding for three of the five years prior to the sugar review.
The FDA's instructions were clear: To label a substance as a potential health hazard, there had to be "credible evidence of, or reasonable grounds to suspect, adverse biological effects"—which certainly existed for sugar at the time. But the GRAS committee's review would depend heavily on "Sugar in the Diet of Man" and other work by its authors. In the section on heart disease, committee members cited 14 studies whose results were "conflicting," but 6 of those bore industry fingerprints, including Francisco Grande's chapter from "Sugar in the Diet of Man" and 5 others that came from Grande's lab or were otherwise funded by the sugar industry.
The diabetes chapter of the review acknowledged studies suggesting that "long term consumption of sucrose can result in a functional change in the capacity to metabolize carbohydrates and thus lead to diabetes mellitus," but it went on to cite five reports contradicting that notion. All had industry ties, and three were authored by Ed Bierman, including his chapter in "Sugar in the Diet of Man."
In January 1976, the GRAS committee published its preliminary conclusions, noting that while sugar probably contributed to tooth decay, it was not a "hazard to the public." The draft review dismissed the diabetes link as "circumstantial" and called the connection to cardiovascular disease "less than clear," with fat playing a greater role. The only cautionary note, besides cavities, was that all bets were off if sugar consumption were to increase significantly. The committee then thanked the Sugar Association for contributing "information and data." (Tatem would later remark that while he was "proud of the credit line...we would probably be better off without it.")
The committee's perspective was shared by many researchers, but certainly not all. For a public hearing on the draft review, scientists from the USDA's Carbohydrate Nutrition Laboratory submitted what they considered "abundant evidence that sucrose is one of the dietary factors responsible for obesity, diabetes, and heart disease." As they later explained in the American Journal of Clinical Nutrition, some portion of the public—perhaps 15 million Americans at that time—clearly could not tolerate a diet rich in sugar and other carbohydrates. Sugar consumption, they said, should come down by "a minimum of 60 percent," and the government should launch a national campaign "to inform the populace of the hazards of excessive sugar consumption." But the committee stood by its conclusions in the final version of its report presented to the FDA in October 1976.
For the sugar industry, the report was gospel. The findings "should be memorized" by the staff of every company associated with the sugar industry, Tatem told his membership. "In the long run," he said, the document "cannot be sidetracked, and you may be sure we will push its exposure to all corners of the country."
The association promptly produced an ad for newspapers and magazines exclaiming "Sugar is Safe!" It "does not cause death-dealing diseases," the ad declared, and "there is no substantiated scientific evidence indicating that sugar causes diabetes, heart disease or any other malady...The next time you hear a promoter attacking sugar, beware the ripoff. Remember he can't substantiate his charges. Ask yourself what he's promoting or what he is seeking to cover up. If you get a chance, ask him about the GRAS Review Report. Odds are you won't get an answer. Nothing stings a nutritional liar like scientific facts."
The Sugar Association would soon get its chance to put the committee's sugar review to the test. In 1977, McGovern's select committee—the one that had held the 1973 hearings on sugar and diabetes—blindsided the industry with a report titled "Dietary Goals for the United States," recommending that Americans lower their sugar intake by 40 percent (PDF). The association "hammered away" at the McGovern report using the GRAS review "as our scientific Bible," Tatem told sugar executives.
McGovern held fast, but Big Sugar would prevail in the end. In 1980, when the USDA first published its own set of dietary guidelines, it relied heavily on a review written for the American Society of Clinical Nutrition by none other than Bierman, who used the GRAS committee's findings to bolster his own. "Contrary to widespread opinion, too much sugar does not seem to cause diabetes," the USDA guidelines concluded. They went on to counsel that people should "avoid too much sugar," without bothering to explain what that meant.
In 1982, the FDA once again took up the GRAS committee's conclusion that sugar was safe, proposing to make it official. The announcement resulted in a swarm of public criticism, prompting the agency to reopen its case. Four years later, an agency task force concluded, again leaning on industry-sponsored studies, that "there is no conclusive evidence...that demonstrates a hazard to the general public when sugars are consumed at the levels that are now current." (Walter Glinsmann, the task force's lead administrator, would later become a consultant to the Corn Refiners Association, which represents producers of high-fructose corn syrup.)
The USDA, meanwhile, had updated its own dietary guidelines. With Fred Stare now on the advisory committee, the 1985 guidelines retained the previous edition's vague recommendation to "avoid too much" sugar but stated unambiguously that "too much sugar in your diet does not cause diabetes." At the time, the USDA's own Carbohydrate Nutrition Laboratory was still generating evidence to the contrary and supporting the notion that "even low sucrose intake" might be contributing to heart disease in 10 percent of Americans.
By the early 1990s, the USDA's research into sugar's health effects had ceased, and the FDA's take on sugar had become conventional wisdom, influencing a generation's worth of key publications on diet and health. Reports from the surgeon general and the National Academy of Sciences repeated the mantra that the evidence linking sugar to chronic disease was inconclusive, and then went on to equate "inconclusive" with "nonexistent." They also ignored a crucial caveat: The FDA reviewers had deemed added sugars—those in excess of what occurs naturally in our diets—safe at "current" 1986 consumption levels. But the FDA's consumption estimate was 43 percent lower than that of its sister agency, the USDA. By 1999, the average American would be eating more than double the amount the FDA had deemed safe—although we have cut back by 13 percent since then.
